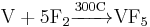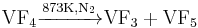Vanadium(V) fluoride
| Vanadium(V) fluoride | |
|---|---|
|
Vanadium(V) fluoride |
|
|
Other names
Vanadium pentafluoride |
|
| Identifiers | |
| CAS number | 7783-72-4 |
| PubChem | 165641 |
| Properties | |
| Molecular formula | VF5 |
| Molar mass | 145.934 |
| Appearance | colorless solid |
| Density | 2.502 g/cm3 (solid) |
| Melting point |
19.5 °C |
| Boiling point |
48.3 °C |
| Related compounds | |
| Other cations | Niobium(V) fluoride Tantalum(V) fluoride |
| Related Vanadium compounds | Vanadium(V) oxide |
| (verify) (what is: /?) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Vanadium(V) fluoride is an inorganic compound with the chemical formula VF5.
It can be prepared by the reaction below:
References
- ^ Trevorrow, L. E.; Fischer, J.; Steunenberg, R. K. (1957). "The Preparation and Properties of Vanadium Pentafluoride". Journal of the American Chemical Society 79 (19): 5167–5168. doi:10.1021/ja01576a023.
- ^ Ruff, Otto; Lickfett, Herbert (1911). "Vanadinfluoride". Berichte der deutschen chemischen Gesellschaft 44 (3): 2539–2549. doi:10.1002/cber.19110440379.
- ^ Cavell, R. G.; Clark, H. C. (1963). "Thermochemistry of vanadium fluorides". Transactions of the Faraday Society 59: 2706. doi:10.1039/TF9635902706.
|
|||||